Navigating the Future: A Comprehensive Guide to the 2026 USP Calendar
Related Articles: Navigating the Future: A Comprehensive Guide to the 2026 USP Calendar
Introduction
In this auspicious occasion, we are delighted to delve into the intriguing topic related to Navigating the Future: A Comprehensive Guide to the 2026 USP Calendar. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
Navigating the Future: A Comprehensive Guide to the 2026 USP Calendar
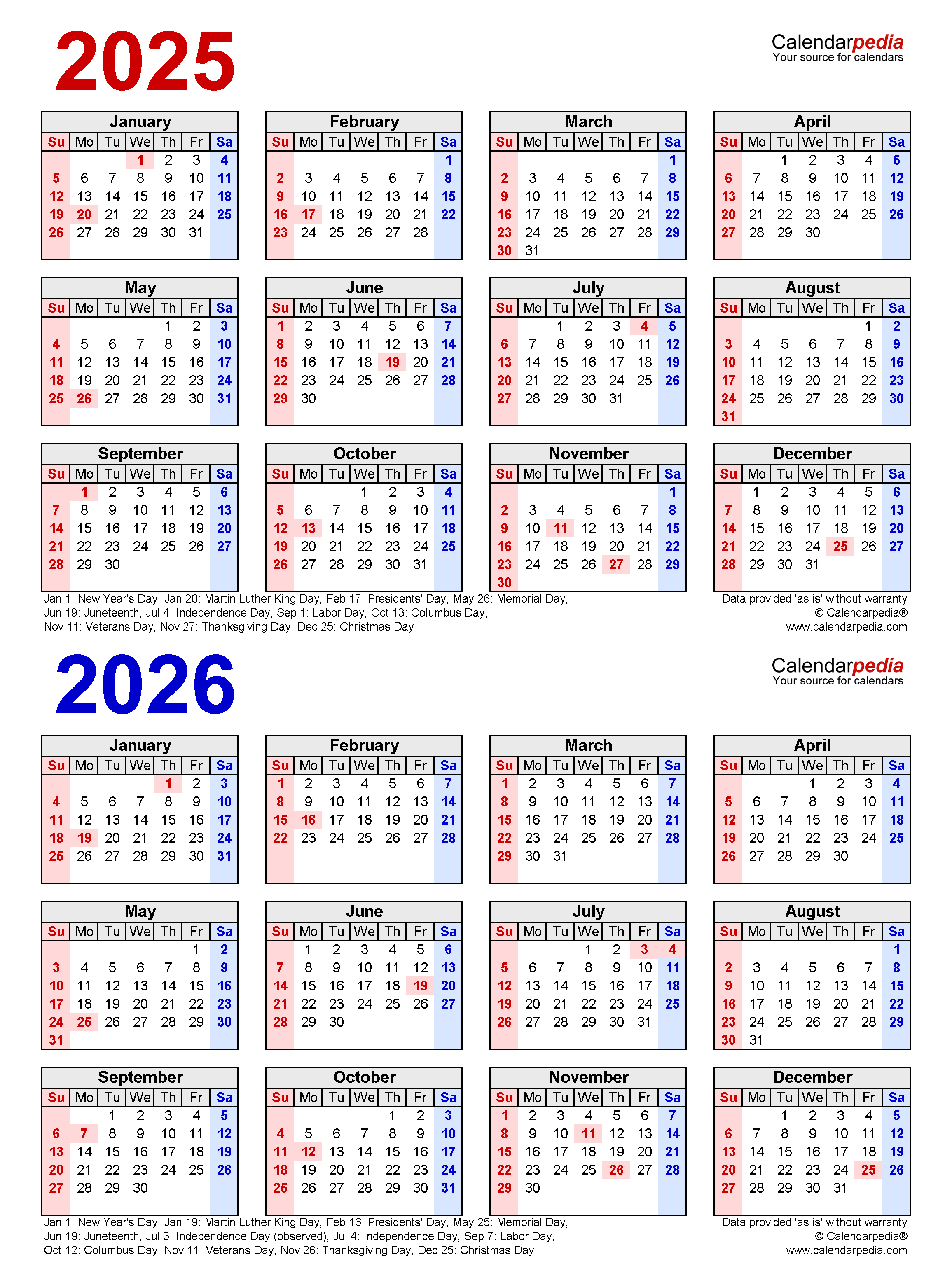
The year 2026 marks a significant milestone in the pharmaceutical landscape. It is the year when the United States Pharmacopeia (USP) will implement a comprehensive revision of its General Chapters, impacting the quality standards and testing procedures for pharmaceutical products. This revision, known as the 2026 USP General Chapters, signifies a critical shift in the industry, demanding proactive adaptation and preparedness from all stakeholders.
This article aims to provide a comprehensive overview of the 2026 USP General Chapters, outlining its key features, benefits, and implications for various stakeholders. It will delve into the rationale behind this revision, the specific changes it introduces, and the potential impact on the pharmaceutical ecosystem. Additionally, the article will address frequently asked questions and provide practical tips for navigating this transition effectively.
Understanding the Need for Revision
The USP General Chapters serve as the foundation for pharmaceutical quality standards in the United States. They provide a comprehensive set of analytical and manufacturing guidelines, ensuring the safety, efficacy, and quality of medications. These chapters are regularly reviewed and updated to reflect advancements in technology, scientific understanding, and evolving regulatory requirements.
The 2026 USP General Chapters represent a significant update, driven by several factors:
- Advancements in Technology: The pharmaceutical industry has witnessed rapid advancements in analytical techniques, instrumentation, and manufacturing processes. The 2026 revision incorporates these advancements, ensuring that the General Chapters remain relevant and aligned with modern practices.
- Global Harmonization: The need for harmonization of pharmaceutical standards across different regions is paramount. The 2026 revision aligns with global initiatives, fostering a more consistent and streamlined approach to quality assurance.
- Evolving Regulatory Landscape: Regulatory frameworks are constantly evolving to address emerging challenges and ensure patient safety. The 2026 revision reflects these changes, incorporating new requirements and guidelines for drug development, manufacturing, and testing.
Key Changes Introduced by the 2026 USP General Chapters
The 2026 revision introduces significant changes across various aspects of pharmaceutical quality control, including:
- Analytical Techniques: The revision incorporates new and improved analytical methods, such as high-performance liquid chromatography (HPLC) and mass spectrometry (MS), for enhanced precision and accuracy in drug analysis.
- Microbial Testing: The General Chapters will include updated guidelines for microbial testing, incorporating new technologies and methodologies for detecting and identifying microbial contaminants.
- Manufacturing Processes: The revision introduces new guidelines for manufacturing processes, focusing on process validation, control strategies, and risk management to ensure consistent product quality.
- Packaging and Labeling: The 2026 revision includes updated requirements for packaging and labeling, addressing issues related to tamper-evident packaging, product identification, and information disclosure.
- Stability Testing: The General Chapters will incorporate new guidelines for stability testing, emphasizing long-term stability studies and real-time monitoring of drug degradation.
Impact of the 2026 USP General Chapters
The 2026 USP General Chapters will have a significant impact on various stakeholders in the pharmaceutical industry:
- Pharmaceutical Manufacturers: Manufacturers will need to adapt their analytical methods, manufacturing processes, and quality control procedures to comply with the new requirements. This will require investments in technology, training, and validation studies.
- Contract Research Organizations (CROs): CROs will need to update their testing protocols and analytical capabilities to meet the new standards. They will also need to ensure their personnel are adequately trained on the revised General Chapters.
- Regulatory Agencies: Regulatory agencies will use the 2026 USP General Chapters as a benchmark for evaluating drug applications and ensuring compliance with quality standards.
- Patients: The 2026 revision aims to improve patient safety and ensure the quality and efficacy of medications. By adhering to the updated standards, manufacturers can provide patients with higher-quality drugs.
Frequently Asked Questions (FAQs)
1. What is the timeline for implementing the 2026 USP General Chapters?
The implementation timeline for the 2026 USP General Chapters is as follows:
- 2023-2024: Public comment period for proposed revisions.
- 2025: Finalization of the revised General Chapters.
- 2026: Effective date for implementation.
2. How can manufacturers prepare for the 2026 USP General Chapters?
Manufacturers should take a proactive approach to prepare for the 2026 USP General Chapters:
- Monitor USP Updates: Stay informed about the proposed revisions and their implications for their products.
- Review Existing Procedures: Evaluate current analytical methods, manufacturing processes, and quality control procedures against the proposed revisions.
- Plan for Upgrades: Identify areas where upgrades or investments are required to comply with the new standards.
- Train Personnel: Ensure that personnel are adequately trained on the revised General Chapters and new analytical techniques.
- Validate Procedures: Conduct validation studies to ensure that existing procedures meet the new requirements.
3. What are the benefits of the 2026 USP General Chapters?
The 2026 USP General Chapters offer several benefits:
- Enhanced Patient Safety: Improved analytical techniques and manufacturing processes contribute to higher-quality medications and improved patient safety.
- Global Harmonization: Alignment with international standards fosters a more consistent and efficient approach to pharmaceutical quality assurance.
- Technological Advancement: Incorporation of new technologies and methodologies leads to more accurate and reliable drug analysis and testing.
- Improved Regulatory Compliance: Adherence to the updated General Chapters ensures compliance with regulatory requirements and avoids potential delays or setbacks.
Tips for Navigating the Transition
- Engage with USP: Actively participate in the public comment period for proposed revisions to provide feedback and influence the direction of the updates.
- Collaborate with Industry Partners: Work with other stakeholders, such as suppliers, CROs, and regulatory agencies, to coordinate efforts and ensure a smooth transition.
- Utilize Available Resources: Leverage resources provided by USP, such as training materials, webinars, and guidance documents, to support the implementation process.
- Stay Informed: Continuously monitor updates and announcements from USP to stay abreast of any changes or clarifications.
Conclusion
The 2026 USP General Chapters represent a significant milestone in the evolution of pharmaceutical quality standards. They reflect advancements in technology, evolving regulatory requirements, and a commitment to global harmonization. By proactively embracing these changes, stakeholders can ensure their continued compliance, contribute to patient safety, and drive innovation in the pharmaceutical industry. The 2026 USP General Chapters mark a pivotal moment in the pursuit of excellence in pharmaceutical quality, paving the way for a more robust and secure future for the industry and its beneficiaries.
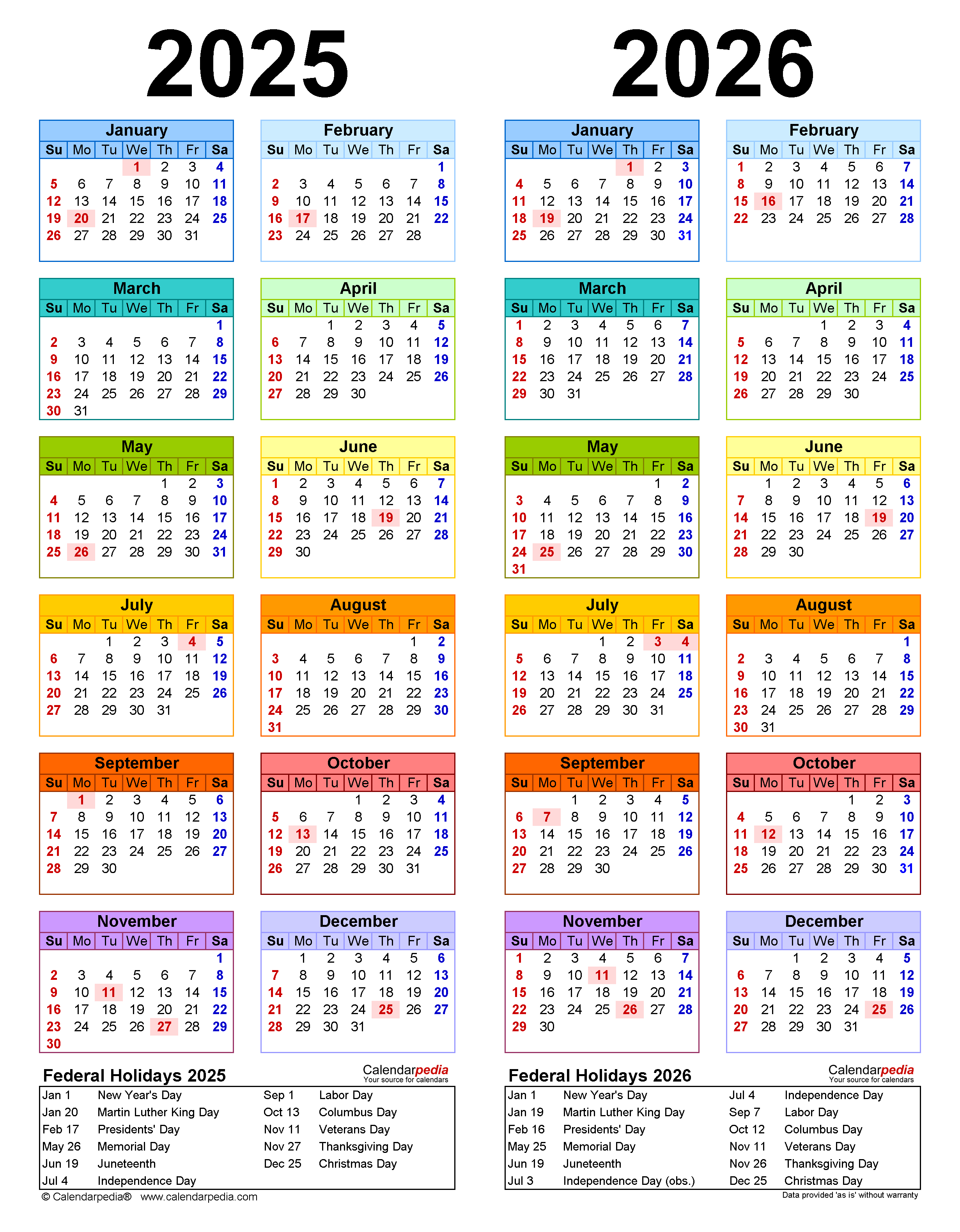
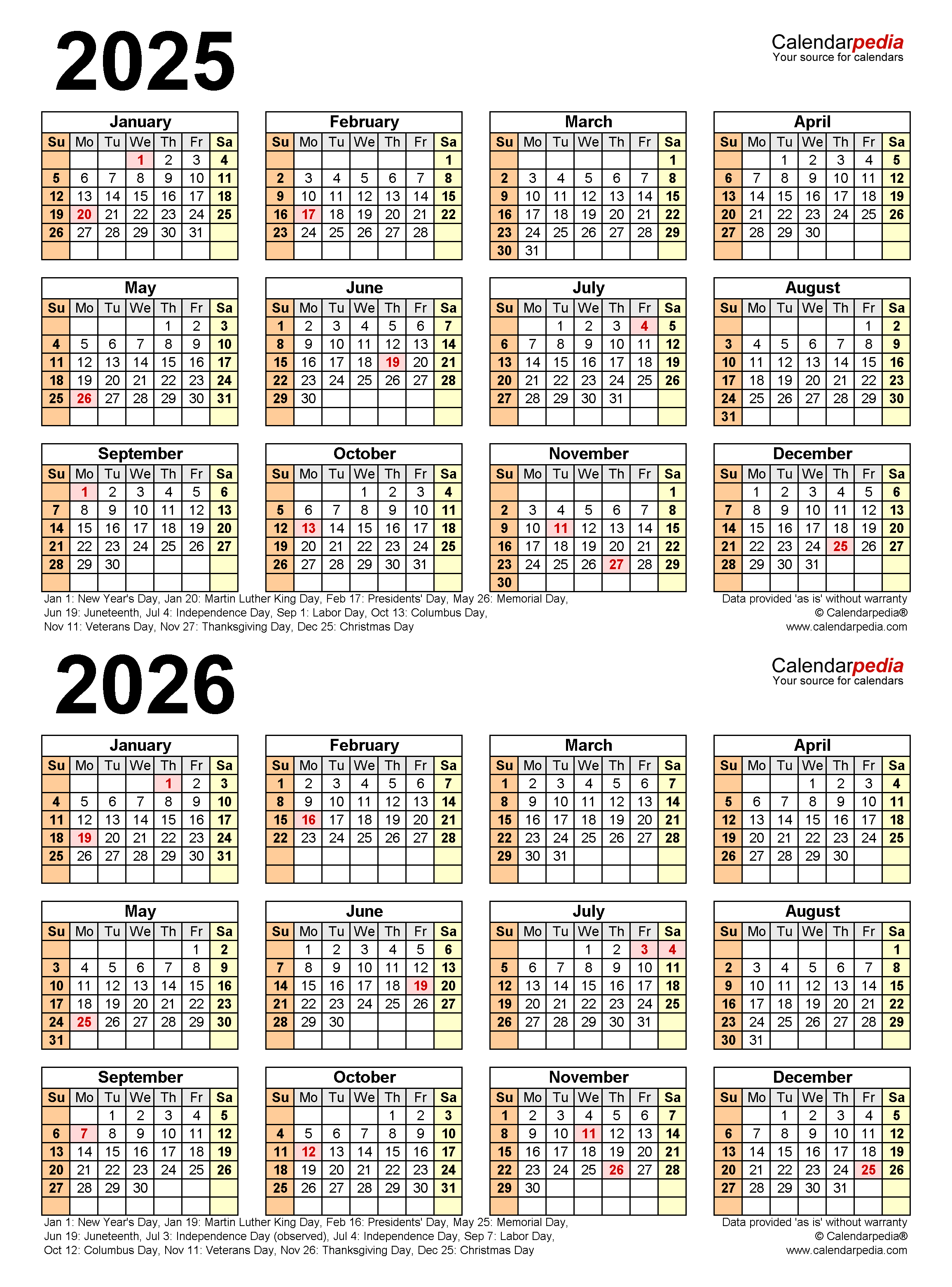
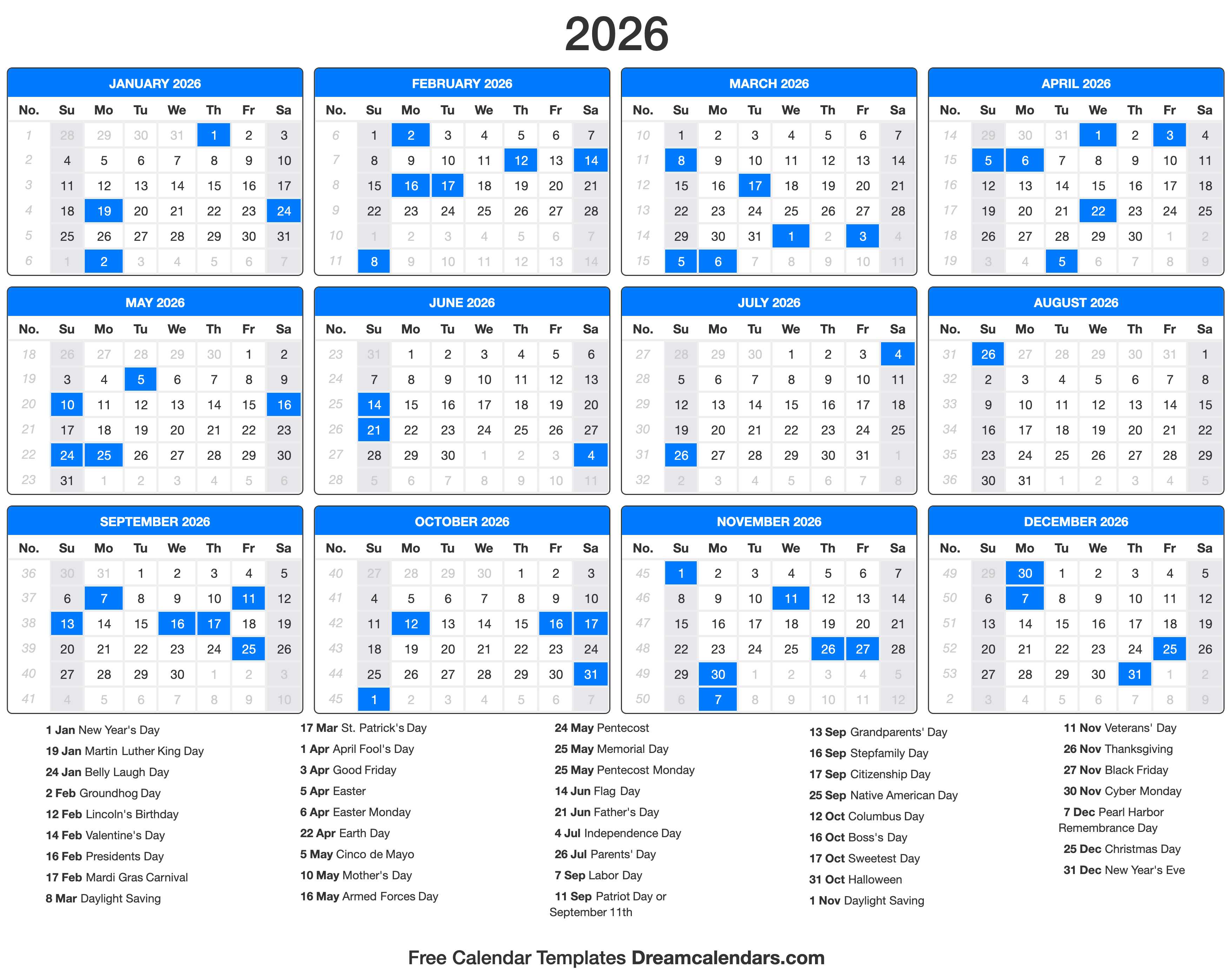
Closure
Thus, we hope this article has provided valuable insights into Navigating the Future: A Comprehensive Guide to the 2026 USP Calendar. We appreciate your attention to our article. See you in our next article!